
Newsroom
Ivy Brain Tumor Center Implements New Liquid Biopsy Program to Provide Glioblastoma Patients More Personalized Care
Innovative procedure offers doctors a scientific snapshot of tumor’s genetic evolution during experimental therapy
PHOENIX, ARIZ., September 7, 2022 – The Ivy Brain Tumor Center at Barrow Neurological Institute, the largest early-phase drug development program for brain cancer, announced its initiation of a ‘Liquid Biopsy’ program to obtain real-time insights into how a patient’s brain tumor is responding to experimental treatment.
Integrating this technique within the Ivy Center’s Phase 0 clinical trial workflow provides doctors with another cutting-edge tool in the fight against brain cancer.
“Early liquid biopsy efforts have focused on using blood or cerebrospinal fluid samples to ascertain tumor diagnosis. We are developing the next-generation of this platform to track tumor evolution in the face of experimental therapy,” said Nader Sanai, MD, Director of the Ivy Brain Tumor Center and Director of Neurosurgical Oncology at Barrow Neurological Institute. “Our strategy is the first of its kind and allows us to follow tumor changes month to month, even when these changes remain undetectable on brain imaging. Since most brain cancers adapt to drug therapies over time, we will uncover these adaptations and look to counteract them with new treatment regimens.”
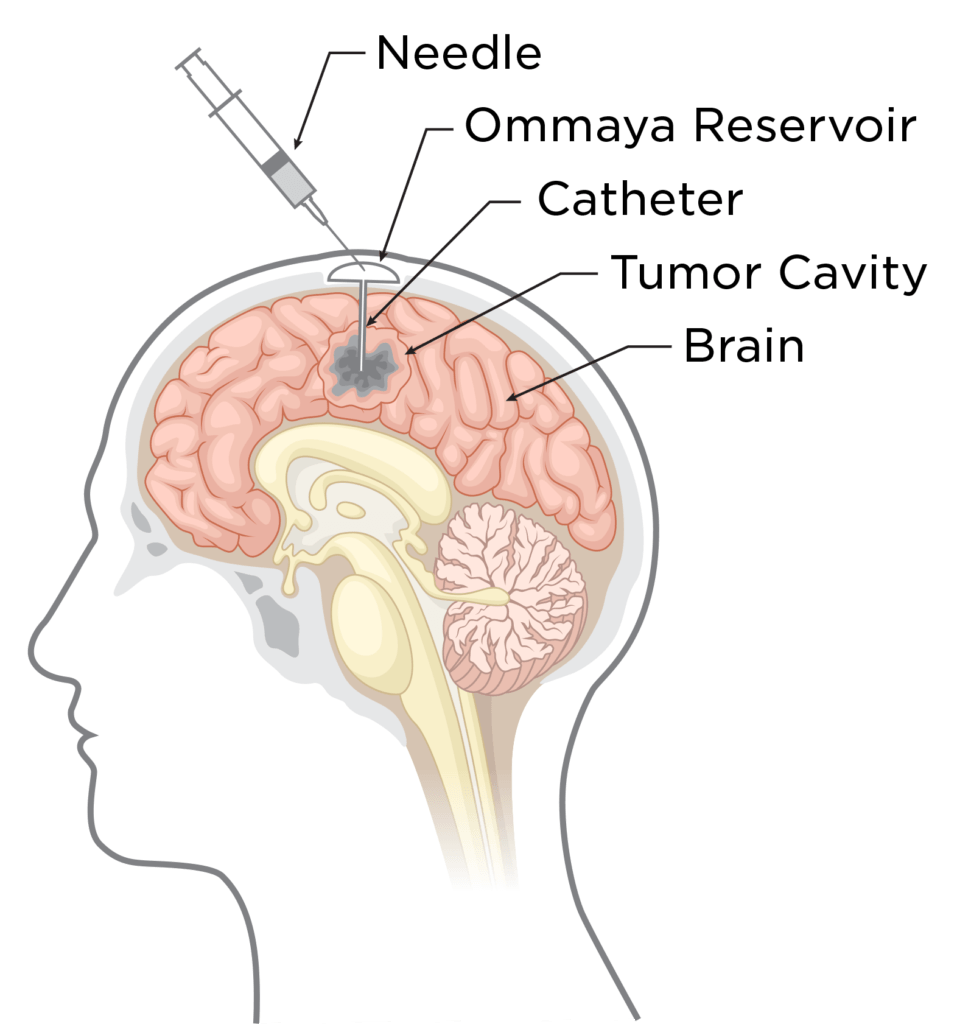
The Ivy Center’s liquid biopsy technique uses a biologically-inert reservoir hidden under the scalp during the patient’s tumor surgery. The reservoir connects to a small catheter that sits within the tumor resection cavity. At routine clinical visits, Ivy Center physicians access the reservoir using a sterile needle and safely extract a small quantity of cerebrospinal fluid (CSF). Tumor-specific genetic material (DNA and RNA) from the CSF is then analyzed and compared to prior samples, identifying potential mechanisms of drug resistance evolving within the tumor cells under the radar.
This new strategy allows scientists and clinical investigators to identify new drug combinations that may counteract resistant brain tumor cells. Because this technique can identify individual tumor cells, it detects tumor even when advanced medical imaging such as MRI does not, enabling action to be taken at the earliest possible opportunity.
This procedure is currently available to participants in the clinical trial of niraparib for newly-diagnosed glioblastoma at the Ivy Brain Tumor Center.
Brain tumors present unique challenges because they are highly complex and heterogeneous, meaning each tumor is slightly different from another. Even cell types within a single tumor are diverse, making them incredibly difficult to treat. Glioblastoma multiforme, or GBM, is the most common type of malignant brain tumor and affects more than 13,000 Americans every year. The prognosis for those struggling with a GBM is grim – nine out of 10 will lose their battle with the disease within five years. GBMs are especially challenging because they have built-in resistance mechanisms against treatment, making a recurrence likely in 70 percent of patients.
The Bottom Line
The Ivy Center’s use of the liquid CSF biopsy lets doctors follow the tumor and track its changes, allowing for quick treatment changes if the current therapy isn’t working. This proactive strategy prevents patients from wasting their precious time, energy, and effort on ineffective therapies.
About Ivy Brain Tumor Center
Ivy Brain Tumor Center at Barrow Neurological Institute in Phoenix, AZ is a non-profit translational research program that employs a bold, early-phase clinical trials strategy to identify new treatments for aggressive brain tumors, including glioblastoma. The Ivy Center’s Phase 0 clinical trials program is the largest of its kind in the world and enables personalized care in a fraction of the time and cost associated with traditional drug development. Unlike conventional clinical trials focusing on single drugs, its accelerated trials program tests therapeutic combinations matched to individual patients. Learn more at IvyBrainTumorCenter.org. Follow the Ivy Brain Tumor Center on Facebook, Instagram, Twitter, and LinkedIn.
